Pupil dilation
From Deliberative Democracy Institiute Wiki
(Redirected from Larning and pupil size)
Contents
Pupillary responce
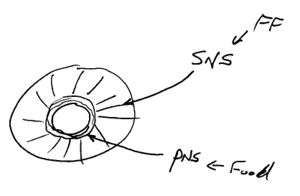
Pupillary response is a physiological response that varies the size of the pupil, via the optic and oculomotor cranial nerve. This response results in either constriction (miosis),[1] narrowing the pupil, or dilation (mydriasis), widening the pupil. Dilation of the pupil occurs when the smooth cells of the radial muscle, controlled by the sympathetic nervous system (SNS), contract. Constriction of the pupil occurs when the circular muscle, controlled by the parasympathetic nervous system (PNS), contracts.
The responses can have a variety of causes, from an involuntary reflex reaction to exposure or inexposure to light — in low light conditions a dilated pupil lets more light into the eye — or it may indicate interest in the subject of attention, or sexual stimulation[2] and is influanced by oxytocine level[3]. The pupils contract immediately before someone falls asleep[4]. A pupillary response can be intentionally conditioned as a Pavlovian response to some stimuli[5].
The latency of pupillary response (the time in which it takes to occur) increases with age[6]. Use of central nervous system stimulant drugs and some hallucinogenic drugs can cause dilation of the pupil[7].
In ophthalmology, intensive studies of pupillary response are conducted via videopupillometry[8].
Anisocoria is the condition of one pupil being more dilated than the other.
| Constriction | Dilation | |
|---|---|---|
| Muscular mechanism | Relaxation of iris dilator muscle and/or activation of the circular muscle |
Activation of iris dilator muscle and/or relaxation of the circular muscle |
| Cause in pupillary light reflex | Increased light | Decreased light |
| Other physiological causes | Accommodation reflex | Fight-or-flight response |
| Corresponding non-physiological state | Miosis | Mydriasis |
Task-invoked pupillary response
From Wikipedia, the free encyclopedia
Task-invoked pupillary response (also known as the "Task-Evoked pupillary response" is a pupillary response caused by a cognitive load imposed on a human and as a result of the decrease in parasympathetic activity in the peripheral nervous system.[9] It is found to result in a linear increase in pupil dilation as the demands a task places on the working memory increase. Beatty, J.[10] evaluated task-invoked pupillary response in different tasks for short-term memory, language processing, reasoning, perception, sustained attention and selective attention and found that it fulfils Kahneman’s[11] three criteria for indicating processing load. That is, it can reflect differences in processing load within a task, between different tasks and between individuals. It is used as an indicator of cognitive load levels in psychophysiology research.
Mechanism
Pupil response is governed by the autonomic nervous system. Pupil dilation depends on the activation of the adrenergic sympathetic nervous system, while pupil constriction depends on the cholinergic parasympathetic nervous system[12]. It has been well documented that pupil response is modulated not only by an ambient luminance level (the so-called pupil light reflex) but also by the amount of mental effort invested in a task [13][14][15][16][17][18][19][20][21][22][23]. For example, Porter et al[24] reported that the pupil dilates when subjects conduct a difficult visual search task in which high mental effort has to be invested.
Neuroadrenaline may play a role in pupulary responce during suprise[25]
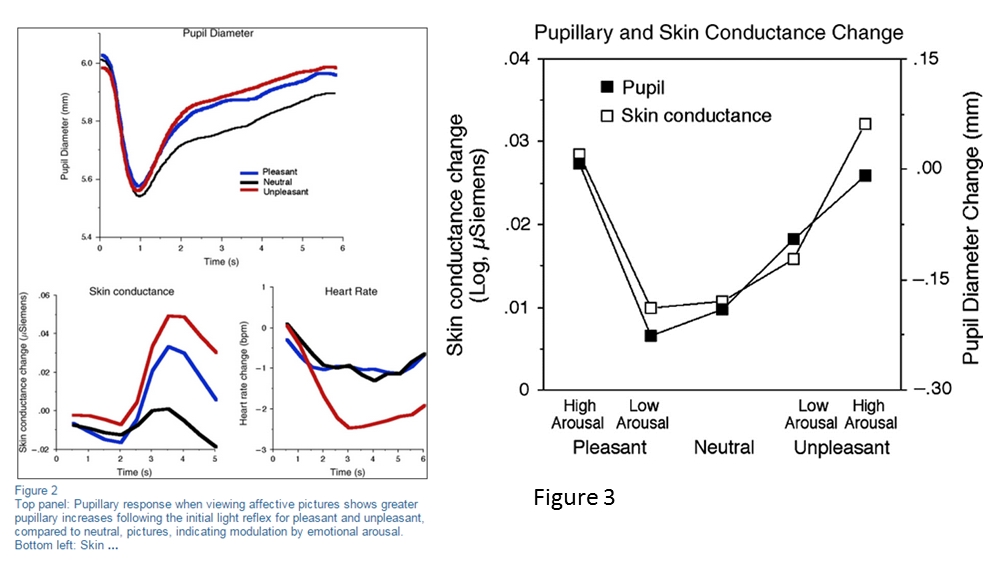
.Emotional Responce
Emotional pictures arouse the SNS which cause mydriasis[26].
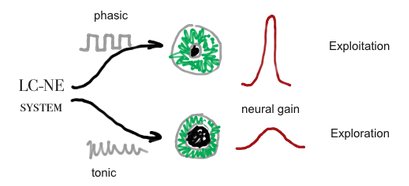
Neural Gain
Converging evidence suggests that baseline pupil diameter is correlated with tonic levels of LC-NE activity in rats, cats and monkeys[28][29], as with human behaviors that are predicted to be associated with tonic LC-NE activity in a variety of experimental tasks and manipulations[30][31][32][33]
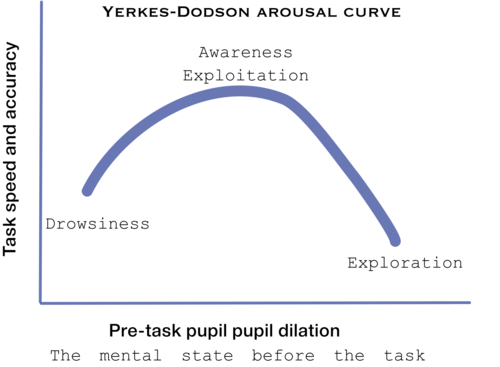
Although, no mechanism was found yet to the connection of pupil size and LC-NE system[34], Murphy et al (2011) found that p300 and pupil dilation is highly corrolated with LC-NE system behviour, according to the Yerkes-Dodson arousal curve[35]:
Pupil size is thought to be controled by the LC-NE system see Cohen[36]and Jepma & Nieuwenhuis[37]. Neural gain, may be the same as mind clouser
Oxitocin enhanced social detecting, and also pupil size (probably because of enhanced learning and attention)[38]
Pupil size enalrgment may be imparid by strong light, this is the reason, Murphy et al. tested the subjects in minimal light[39]
There is a link between ACC activation and pupil size[40]
RPE
RPE can cause pupil dilation in the case of risk error prediction[41][42].
Fear
Intimidating faces can cause pupil dilation[43].
Furteher reading
References
- ↑ Ellis CJ (November 1981). "The pupillary light reflex in normal subjects" (PDF). Br J Ophthalmol 65 (11): 754–9.
- ↑ "Pupil Size as Related to Interest Value of Visual Stimuli", Science 132 (3423), 5 August 1960: 349–50,
- ↑ Leknes, S., Wessberg, J., Ellingsen, D.-M., Chelnokova, O., Olausson, H., & Laeng, B. (2012). Oxytocin enhances pupil dilation and sensitivity to “hidden”emotional expressions. Social Cognitive and Affective Neuroscience, nss062.
- ↑ "Pupillary Movements During Acute and Chronic Fatigue: A New Test for the Objective Evaluation of Tiredness" (PDF), Investigative Ophthalmology (St. Louis: C.V. Mosby Company) 2 (2), April 1963: 138–157
- ↑ Baker, Lynn Erland (1938). "The Pupillary Response Conditioned to Subliminal Auditory Stimuli". Ohio State University.
- ↑ Latency of pupillary reflex to light stimulation and its relationship to aging (PDF), Federal Aviation Agency, Office of Aviation Medicine, Georgetown Clinical Research Institute, September 1965, p. 12,
- ↑ Jaanus SD (1992), "Ocular side effects of selected systemic drugs", Optom Clin 2 (4): 73–96,
- ↑ "A new videopupillography", Ophthalmologica 160 (4), 1970: 248–259, doi:10.1159/000305996, PMID 5439164
- ↑ [1] Kramer, A. F., 1991. Physiological metrics of mental workload: A review of recent progress. In: Damos, D. L. (ed.) Multiple-task Performance. London: Taylor & Francis Ltd.
- ↑ [2] Beatty, J., 1982, Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin, 91, 276-292.
- ↑ [3] Kahneman, D., 1973, Attention and effort, Englewood Cliffs, N. J., Prentice-hall.
- ↑ Barbur J (2004) Learning from the pupil: studies of basic mechanisms and clinical applications. The Visual Neurosciences Vol. 1. In: Chlupa L, Werner J, editors. Cambridge: The MIT Press. pp. 641–656.
- ↑ Beatty J (1982) Task-evoked papillary responses, processing load, and the structure of processing resources. Psychological Bulletin 91: 276–292. doi: 10.1037/0033-2909.91.2.276
- ↑ Hampson RE, Opris I, Deadwyler SA (2010) Neural correlates of fast pupil dilation in nonhuman primates: relation to behavioral performance and cognitive workload. Behavioral Brain Research 212: 1–11.
- ↑ Kahneman D, Beatty J (1966) Pupil diameter and load on memory. Science 154: 1583–1585. doi: 10.1126/science.154.3756.1583
- ↑ Kahneman D, Peavler W (1969) Incentive effects and papillary changes in association learning. Journal of Experimental Psychology 79: 312–318.
- ↑ Partala T, Surakka V (2003) Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies 59: 185–198. doi: 10.1073/pnas.88.11.4966
- ↑ Porter G, Troscianko T, Gilchrist I (2007) Effort during visual search and counting: insights from pupilometry. The Quarterly Journal of Experimental Psychology 60: 211–229. doi: 10.1080/17470210600673818
- ↑ van Orden KF, Jung T-P, Makeig S (2000) Combined eye activity measures accurately estimate changes in sustained visual task. Biological Psychology 52: 221–240. doi: 10.1016/S0301-0511(99)00043-5
- ↑ van Orden KF, Limbert W, Makeig S, Jung T-P (2001) Eye activity correlates of workload during visuospatial memory task. Human Factors 43: 111–121. doi: 10.1518/001872001775992570
- ↑ Heaver, Becky, and Sam B. Hutton. "Keeping an eye on the truth? Pupil size changes associated with recognition memory." Memory 19.4 (2011): 398-405.
- ↑ Silvetti, Massimo, et al. "The influence of the noradrenergic system on optimal control of neural plasticity." Frontiers in behavioral neuroscience 7 (2013).
- ↑ Takeuchi, Tatsuto, et al. "Estimation of Mental Effort in Learning Visual Search by Measuring Pupil Response." PloS one 6.7 (2011): e21973.
- ↑ Porter G, Troscianko T, Gilchrist I (2007) Effort during visual search and counting: insights from pupilometry. The Quarterly Journal of Experimental Psychology 60: 211–229. doi: 10.1080/17470210600673818
- ↑ [ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3183372/ Preuschoff, K., Marius’t Hart, B., & Einhäuser, W. (2011). Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Frontiers in Neuroscience, 5.]
- ↑ Bradley, M. M., Miccoli, L., Escrig, M. A., & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607.
- ↑ Bradley, M. M., Miccoli, L., Escrig, M. A., & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607.
- ↑ Aston-Jones, G. & Cohen, J.D. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
- ↑ Koss, M.C. Pupillary dilation as an index of central nervous system α2-adrenoceptor activation. J. Pharmacol. Methods 15, 1–19 (1986).
- ↑ Gilzenrat, M.S., Nieuwenhuis, S., Jepma, M. & Cohen, J.D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 10, 252–269 (2010).
- ↑ Jepma, M. & Nieuwenhuis, S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J. Cogn. Neurosci. 23, 1587–1596 (2011).
- ↑ Einhäuser, W., Stout, J., Koch, C. & Carter, O.L. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. USA 105, 1704–1709 (2008).
- ↑ Murphy, P.R., Robertson, I.H., Balsters, J.H. & O'Connell, R.G. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology 48, 1532–1543 (2011).
- ↑ Nieuwenhuis, S., De Geus, E. J., & Aston-Jones, G. (2011). The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology, 48(2), 162–175.
- ↑ Murphy, P. R., Robertson, I. H., Balsters, J. H., & O’connell, R. G. (2011). Pupillometry and P3 index the locus coeruleus--noradrenergic arousal function in humans. Psychophysiology, 48(11), 1532–1543.
- ↑ Eldar, Eran, Jonathan D Cohen & Yael Niv. 2013. The effects of neural gain on attention and learning. Nature Neuroscience. Published online June 16, 2013 doi:10.1038/nn.3428 (summery)
- ↑ Marieke Jepma and Sander Nieuwenhuis, 2011, Pupil Diameter Predicts Changes in thev Exploration – Exploitation Trade-off: Evidence for the Adaptive Gain Theory, Jurnal of Cognitive Neuroscience 23:7, pp. 1587-1596
- ↑ Siri Leknes, Johan Wessberg, Dan-Mikael Ellingsen, Olga Chelnokova1, Håkan Olausson and Bruno Laeng (2012) Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions
- ↑ see at the discussion section: Murphy, P. R., Robertson, I. H., Balsters, J. H., & O’connell, R. G. (2011). Pupillometry and P3 index the locus coeruleus--noradrenergic arousal function in humans. Psychophysiology, 48(11), 1532–1543.
- ↑ Critchley, HD, Tang, J, Glaser, D, Butterworth, B, Dolan, RJ (2005) Anterior cingulate activity during error and autonomic response
- ↑ Preuschoff, K., Marius’t Hart, B., & Einhäuser, W. (2011). Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Frontiers in neuroscience, 5
- ↑ Satterthwaite, T. D., Green, L., Myerson, J., Parker, J., Ramaratnam, M., & Buckner, R. L. (2007). Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. Neuroimage, 37(3), 1017–1031.
- ↑ Tomer from Haifa, 2015, in publication